
Spectrophotometry,what is Spectrophotometry? uses of Spectrophotometry? types of Spectrophotometry?
Spectrophotometry
spectrum of light
light is supposed to have a dual characteristics corpuscular and wave form. thus a beam of light has an Electromagnetic wave form a {or} photon of energy propagated at 3×108m/sec at the speed of light.
The term Electromagnetic is precise description of the radiation in that radiation is made up of an Electric and a magnetic wave which are perpendicular to each other.
the magnitude of electrical vector is denoted by symbol E and that of electrical vector is the symbol h the distance along the direction of propagation for one complete cycle is known as wavelength.
wave length may be measured in centimeters,micrometes, nanometers, A0.
sometimes a term frequency is used rather than wavelength to describe a particular radiation. frequency share and Inverse relationship with the wavelength.

sometimes radiation is characterized by another term known as wave number denoted by the symbol v wave number means the number of complete cycles occurring per centimeter.
| Planck’s Constant. |
the energy E of a photon can be related to its wavelength and frequency with the help of Planck’s Constant.
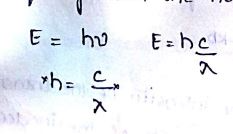
absorption of electromagnetic radiation
the absorption of light by any absorbing materials is governed by two laws.
the first of these laws is known as “burger Lambert law.” it states that the amount of light observed is proportional to the thickness of absorbing material and is independent of the intensity of light.
let us assume that a thickness ‘b’ has the ability to absorb 50% of the incident intensity of the light passing through it.
If the intensity of the radiation incident upon such a thickness is assigned a value of 1.0 the out coming that has the transmitted beam will have a value of 0.5 if we now place a second equal thickness ‘b’ it will absorb 50% of transmitted beam that have 50% of 0.5 the second transmitted beam will then have a value 0.25.
| absorption of electromagnetic radiation |
Beers law :-
the amount of light observed is proportional to the thickness of the absorbent material and is independent of the intensity of the incident light. the successive light intensities {or} the sequence
(0.5)1 (0.5)2 (0.5)3 etc,.this is clearly an expotential function and may be expressed as
I =the intensity of transmitted light
I0 =the intensity of incident light
B =the adsorbent thickness {or} path length
K =the linear exception of coefficient of the absorbing material.
The power turn in the above relationship can be removed by converting to the logarithmic form.
Beer’s law states that the amount of light absorb by a material is proportional to the number of absorbing molecules that have the concentration of absorbing solution. this can be mathematically expressed in the form of an equation
we can combine two equals bougher- Lambert law and Beer’s law here K and K1 merge to become a single constant. the combined equation is written as
| light absorb by a material |
this equation has been alternative referred to as beer Lambert law Deviations:-
| deviations from beer’s law |
deviations from beer’s law occurs as usually when high sample concentrations are being measured. One effect of high concentration is that the molecules may dimerised. it is not necessary that the absorption spectrum of the dimmers are the same as that of monomers.
if the spectra differ the absorption Coefficient the abs will also undergo a change leading to a positive or negative deviation.
high concentration may also lead to aggregation large aggregates than scatter light.
aggregation can lead to Electronic interactions that can either lessen enhance the absorption Coefficient.
high concentration can also lead to chemical reactions which will lead to a change in the chemical composition of the solution. Deviations may also occur at low concentration proteins are known to denature at low concentration and the denature product has an absorption spectrum that is different from the native protein.
Instrumentation limitations may also result in the deviations from beer’s law one of the main cause here is imperfect monochromacy.
| concentration and extinction |
extinction Coefficient:-
absorption Coefficient is also known as extinction coefficient.
| extinction Coefficient |
The numerical value of a depends on the units in which c and B are expressed if the concentration is given in g/it a becomes the specific absorption Coefficient ąs if c is expressed in M/IT and is expressed in centimetres the coefficient am and is known as “molar extinction coefficient” or “molar absorptivity constant”
It is a physical contant.
Am = as × mw
Am = A/cb [A=absorbance]
Instrumentation for uv-visible spectrophotometric:
The instrument that are used to study the adsorption (or) less similar optical principles are employed in these instruments. The essential components of a spectrophotometer include:
A stable and cheap radiant energy source.
A mono-chromatin to break the polychromatin radiation in to component wave length.
Transparent vessels to hold the samples.
A photosensitive detector and associated read out system.
Radiant energy source:
Source of uv radiation
Most of uv radiation are hydrogen lamp and deuterium lamp.
Both the systems consist of a pair of electrodes enclosed in a glass tube provided with a quartz window.
The glass tube is filled with hydrogen or deuterium gas at low pressure.
When a stabilized high voltage is applied they emit radiation which is continuous in the region roughly 180 and 35 km.
Xenon lamp may also be used for cultravoilet radiation, but the radiation produced is not as stable as hydrogen lamp.
Source of visible-radiation:
Tungsten filament lamp is the most commonly used source for ‘v’
Radiation.
It emits continuous in the region between 350 and 2500nm.
Wavelength selectors are 2 types: 1) filters and 2)monochromators.
1) filters:-
filters operate by observing light in all other region except for which they reflect.
Gelatin filters are made of a layer of gelatin, coloured with organic dies and sealed between glass plate.
2)monochromators:-
A monochromator resovles polychromatic radiation in to
Its individual wavelength and isolates these wavelength into very narrow bands. The essential components of a monochromator are.
An entrencetics which admit polychromatic light.
A collimating device such as lens (or) a mirror which collimates the polychromatic light on to the despension device.
A wave length resolving device like a prism.
A focusing lens.
An existing slit which allows the monochromatic beam to escape.
| monochromatic beam to escape |
Sample containers:
Most of the spectrophotometric studies are made in solution.
The solution are dispensed in cells known as cuvettes is usually 1 cm. the surface of cuvettes must be kept clean, finger print smudges and traces of previous samples by causing interference in the optical path might cause serious errors in quantitative measurements.
Detection devices:
There are three basic kinds of electors- photo cells, phototubes and photo multipliers.
Photovoltaic cell :-
A typical photo cell consist of a thin transparent silver film on a stell base.
This arrangment ensures ensures that electrons pass easily from selenium to silver but not in the reverse direction silver acts as acts as collecting electrode for electrons and steel plate functions as the other electrode.
| Photovoltaic cell |
Phototube:-
The components of a Phototube include an evaculated glass envelope with a quartz window. The semi cylindrical cathode whose inner surface is coated with alkali a centrally located metal wire anode. A potential difference of approx. 90 v is applied across the electrode. If the ē collection is 100% efficient the phototube current should be propotional to the light intensity.
| Phototube |
Photomultipliers:
these detectors are designed to amplify the initial photoelectric affect and are suitable for very low light intensities.
Amplification and read out:
Radiation detectors generate electronic signals which are proportional to the transmitted light. these signals which are proportional to the transmitted light. these signals need to be translated in to a form that is easy to interpret. This is accomplished by using amplifiers, ammeters, potentiometers and potentiometric recorders.
Double- beam operation:
Voltage fluctuations including fluctuations in the source intensity can use large scale errors in spectrophotometer operation. to obviate this situation, Double- beam spectrophotometers have been design
Double beam instruments employee some type of beam splitter prior to the sample containers one of the split beams passes through the blank while the other passes through the sample .the two transmitted beams are then compared either continuously {or} alternately several times in a second. The double beam device therefore compensates for fluctuations in the source intensity, the detectors signal and amplifier gain by absorbing the differences the signal between reference and sample at virtually the same time.
| Photomultipliers |
Dual wavelength spectrophotometer
some metal chelators absorb at a completely different wavelength before chelation has taken place an example is that of arsenazo 3,a calcium chelator. this chelator absorbs at 675 NM before binding to calcium and add 685 nanometer after the binding has taken place.
if this chelator is incubated with a biological system and the absorbance of the chelator is measured simultaneously at the wavelength pair 675 nm, 685 NM the ratio of the two absorbance can provide an idea of the calcium concentration in the given biological system.
dual wavelength spectrophotometry refers to the photo metric measurement by the material by passing radiation of two different wavelengths through the same sample before reaching the detector.
| Dual wavelength spectrophotometer |
Application of UV visible spectrophotometer:-
qualitative analysis
ultraviolet visible spectrum may be used to identify classes of compounds in both pure state and in biological preparations.
this is as usually done by plotting absorption spectrum curves.
obsorption by a compound in different regions gives some hints of its structure. the compound which do not absorb in 220 – 280 nm region are usually aliphatic hydrocarbons or their derivatives.
if the compound absorbs to 220 to 250 nanometres range, it will as usually contain to unsaturated linkage in conjugation.
presence of more than two conjugated double bonds as usually gives rise to Absorption in the range 250 to 330 NM.
as the number of conjugated double bonds increases the absorption range is shifted more and more to higher wavelength.
Β-carotene a precursor of Vitamin A has 11 double bonds in a conjugated system and appears yellow because the light is invisible region [450-500] is being absorbed by it.
quantitative analysis:
in developing a quantitative method for determine an unknown concentration of a given species by absorption spectrometry
most of the organic compounds of biological interest absorb in the UV visible range of spectrum.
nucleic acid at 254 NM and proteins at to 280 nanometres are measured by using UV visible spectrophotometers.
the quantitative assay of enzyme activity is carried out by using UV spectrometry.
molecular weight of the compound can be calculated on the basis of its absorption data. molecular weight of only small molecules may be determined by this method.
absorption spectrophotometry can be used to study sis trans isomerism.
UV visible spectrophotometer has been used to study physico-chemical phenomena.
impurities in a compound can be detected very easily by spectrophotometric studies.
Spectroflurometry:
the phenomenon where by a molecule after observing radiation emits radiation of a longer wavelength is known as fluorescence.
Instrumentation:
flurometry is an important analytical tool for the determination of extremely small concentrations of substance which exhibit fluorescence.
there are two monochromators instead of one as in a spectrophotometer, one monochromator is placed before the sample holder and one after it.
as a fluorescence is maximum between 20,25 to 300c the sample holder has device to maintain the temperature sample.
| Spectroflurometry |
a continuous source of Radiant energy
A monochromator as usually a Prism to choose the wavelength with which the sample is to be a irradiated.
A second monochromator which place after the sample enables the determination of fluorescent spectrum of the sample.
A detected as usually a photomultiplier suited of wavelength greater than 500 nanometres.
an amplifier:
applications:
the more common applications of spectrofluorometry include quantitative and quantitative analysis.
it is used in intracellular free calcium concentration assay.
it is used in fluorescent probes and studied on membrane structure.
assay of membrane potential.
it is used to monitor the Kinetic sand thermodynamics.
absorption and emission flame photometry:-
instrumentation for emission flame photometry :-
the basic component of the flame emission spectrophotometer as follows
| emission flame photometry |
Nebulizer:-
samples before they get into the flame must be converted into a fine spray that has been nebulized.
this is necessary because large drops do not remain in the hottest part of the flame for a long time.
the flame various gas mixtures producing different films differentiating in the temperature are used in flame photometry.
Monochromators:-
in sophisticated instruments prisons or sometimes even refraction gratings are used however for routine analysis of such elements as calcium Sodium and potassium a simple filter might be sufficient.
Photo cells:-
these are the usually detectors in the flame photometer unfortunately the flame instability reduce their accuracy therefore, a multi channel polychromatic is used in some routine procedures to allow measurement of up to 6 elements simultaneously.
instrumentation for absorption spectrophotometry:-
this is practically similar to that of emission flame photometry an important point of differentiation is the need to have a radiation source.
it is particle e practically impossible to isolate a particular region wavelength from continuous source by using a Prism.
this problem was solved by developing a hollow cathode discharge lamp.
such lamp is monochromatic radiation characterized with of the elements analysis.
instruments with single and double beam optics are available.
the double beam optical arrangement is more or less similar to that of double beam apparatus apparatus in absorption spectrophotometry.
Applications:
the primary use of flame photometry and absorption spectrophotometry is in the shape of elements in biological samples such as blood, plasma and other body fluid such as urine, saliva, cerebrospinal fluid and milk.
flame photometry is used in estimation of Sodium Potassium calcium magnesium manganese in and th in host of biological samples.
flame photometry is very sensitive to the estimation of alkali,
alkaline earth and rare Earth elements.
absorption spectrophotometery can detect quantitative less than 1 part 10-6 of more than 20 elements.